Hepatotoxicity: Serious hepatic adverse reactions occurred in 3% of patients treated with Retevmo. Increased aspartate aminotransferase (AST) occurred in 59% of patients, including Grade 3 or 4 events in 11% and increased alanine aminotransferase (ALT) occurred in 55% of patients, including Grade 3 or 4 events in 12%. Monitor ALT and AST prior to initiating Retevmo, every 2 weeks during the first 3 months, then monthly thereafter and as clinically indicated. Withhold, reduce dose, or permanently discontinue Retevmo based on the severity.
Severe, life-threatening, and fatal interstitial lung disease (ILD)/pneumonitis can occur in patients treated with Retevmo. ILD/pneumonitis occurred in 1.8% of patients who received Retevmo, including 0.3% with Grade 3 or 4 events, and 0.3% with fatal reactions. Monitor for pulmonary symptoms indicative of ILD/pneumonitis. Withhold Retevmo and promptly investigate for ILD in any patient who presents with acute or worsening of respiratory symptoms which may be indicative of ILD (e.g., dyspnea, cough, and fever). Withhold, reduce dose, or permanently discontinue Retevmo based on severity of confirmed ILD.
Hypertension occurred in 41% of patients, including Grade 3 hypertension in 20% and Grade 4 in one (0.1%) patient. Overall, 6.3% had their dose interrupted and 1.3% had their dose reduced for hypertension. Treatment-emergent hypertension was most commonly managed with anti-hypertension medications. Do not initiate Retevmo in patients with uncontrolled hypertension. Optimize blood pressure prior to initiating Retevmo. Monitor blood pressure after 1 week, at least monthly thereafter, and as clinically indicated. Initiate or adjust anti-hypertensive therapy as appropriate. Withhold, reduce dose, or permanently discontinue Retevmo based on the severity.
Retevmo can cause concentration-dependent QT interval prolongation. An increase in QTcF interval to >500 ms was measured in 7% of patients and an increase in the QTcF interval of at least 60 ms over baseline was measured in 20% of patients. Retevmo has not been studied in patients with clinically significant active cardiovascular disease or recent myocardial infarction. Monitor patients who are at significant risk of developing QTc prolongation, including patients with known long QT syndromes, clinically significant bradyarrhythmias, and severe or uncontrolled heart failure. Assess QT interval, electrolytes, and thyroid-stimulating hormone (TSH) at baseline and periodically during treatment, adjusting frequency based upon risk factors including diarrhea. Correct hypokalemia, hypomagnesemia, and hypocalcemia prior to initiating Retevmo and during treatment. Monitor the QT interval more frequently when Retevmo is concomitantly administered with strong and moderate CYP3A inhibitors or drugs known to prolong QTc interval. Withhold and dose reduce or permanently discontinue Retevmo based on the severity.
Serious, including fatal, hemorrhagic events can occur with Retevmo. Grade ≥3 hemorrhagic events occurred in 3.1% of patients treated with Retevmo including 4 (0.5%) patients with fatal hemorrhagic events, including cerebral hemorrhage (n=2), tracheostomy site hemorrhage (n=1), and hemoptysis (n=1). Permanently discontinue Retevmo in patients with severe or life-threatening hemorrhage.
Hypersensitivity occurred in 6% of patients receiving Retevmo, including Grade 3 hypersensitivity in 1.9%. The median time to onset was 1.9 weeks (range: 5 days to 2 years). Signs and symptoms of hypersensitivity included fever, rash and arthralgias or myalgias with concurrent decreased platelets or transaminitis. If hypersensitivity occurs, withhold Retevmo and begin corticosteroids at a dose of 1 mg/kg prednisone (or equivalent). Upon resolution of the event, resume Retevmo at a reduced dose and increase the dose of Retevmo by 1 dose level each week as tolerated until reaching the dose taken prior to onset of hypersensitivity. Continue steroids until patient reaches target dose and then taper. Permanently discontinue Retevmo for recurrent hypersensitivity.
Tumor lysis syndrome (TLS) occurred in 0.6% of patients with medullary thyroid carcinoma receiving Retevmo. Patients may be at risk of TLS if they have rapidly growing tumors, a high tumor burden, renal dysfunction, or dehydration. Closely monitor patients at risk, consider appropriate prophylaxis including hydration, and treat as clinically indicated.
Impaired wound healing can occur in patients who receive drugs that inhibit the vascular endothelial growth factor (VEGF) signaling pathway. Therefore, Retevmo has the potential to adversely affect wound healing. Withhold Retevmo for at least 7 days prior to elective surgery. Do not administer for at least 2 weeks following major surgery and until adequate wound healing. The safety of resumption of Retevmo after resolution of wound healing complications has not been established.
Retevmo can cause hypothyroidism. Hypothyroidism occurred in 13% of patients treated with Retevmo; all reactions were Grade 1 or 2. Hypothyroidism occurred in 13% of patients (50/373) with thyroid cancer and 13% of patients (53/423) with other solid tumors including NSCLC. Monitor thyroid function before treatment with Retevmo and periodically during treatment. Treat with thyroid hormone replacement as clinically indicated. Withhold Retevmo until clinically stable or permanently discontinue Retevmo based on severity.
Based on data from animal reproduction studies and its mechanism of action, Retevmo can cause fetal harm when administered to a pregnant woman. Administration of selpercatinib to pregnant rats during organogenesis at maternal exposures that were approximately equal to those observed at the recommended human dose of 160 mg twice daily resulted in embryolethality and malformations. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with Retevmo and for 1 week after the last dose. There are no data on the presence of selpercatinib or its metabolites in human milk or on their effects on the breastfed child or on milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with Retevmo and for 1 week after the last dose.
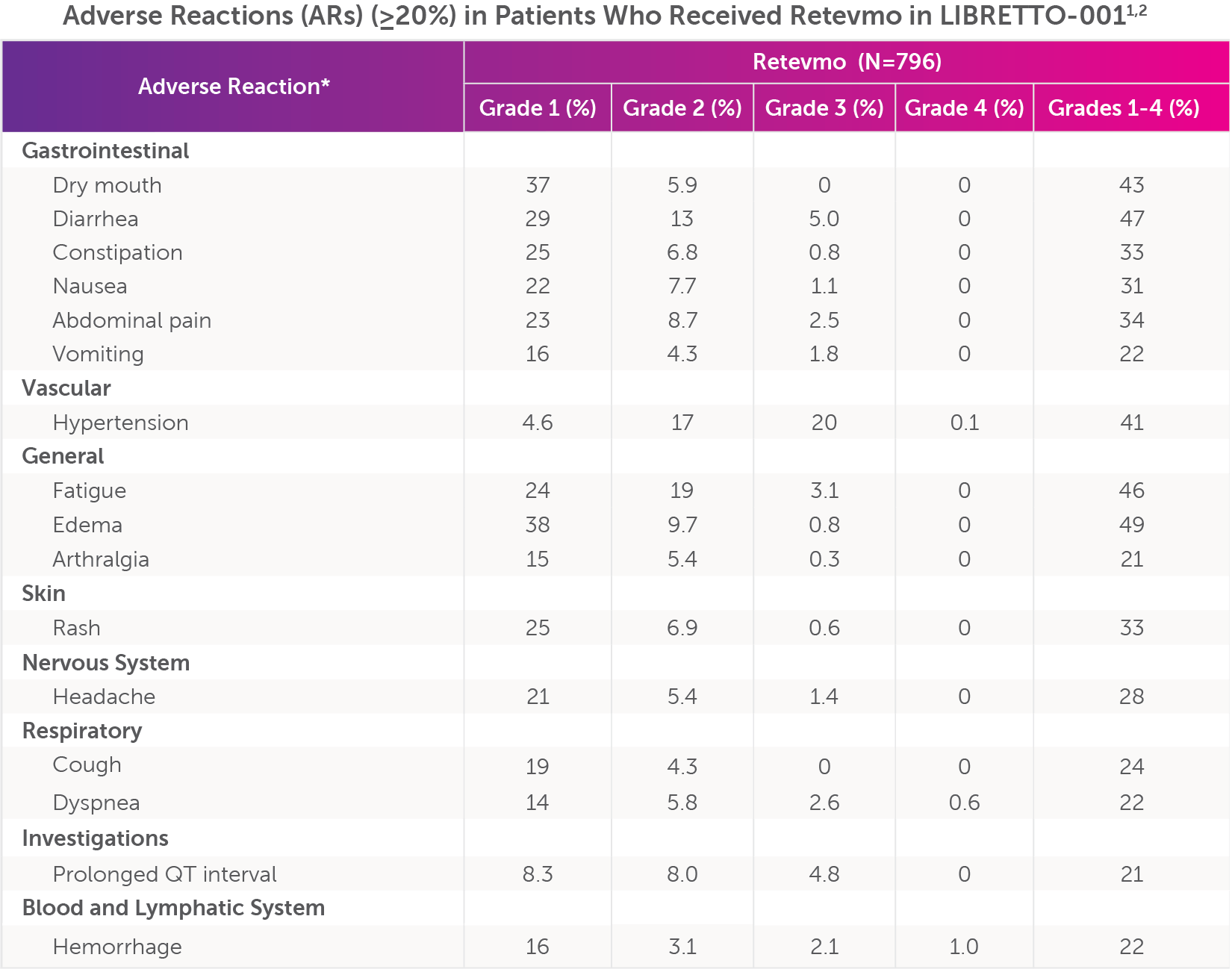
Severe adverse reactions (Grade 3-4) occurring in ≥20% of patients who received Retevmo in LIBRETTO-001, were hypertension (20%), diarrhea (5%), prolonged QT interval (4.8%), dyspnea (3.1%), fatigue (3.1%), hemorrhage (2.6%), abdominal pain (2.5%), vomiting (1.8%), headache (1.4%), nausea (1.1%), constipation (0.8%), edema (0.8%), rash (0.6%), and arthralgia (0.3%).
Serious adverse reactions occurred in 44% of patients who received Retevmo. The most frequently reported serious adverse reactions (in ≥2% of patients) were pneumonia, pleural effusion, abdominal pain, hemorrhage, hypersensitivity, dyspnea, and hyponatremia.
Fatal adverse reactions occurred in 3% of patients; fatal adverse reactions included sepsis (n=6), respiratory failure (n=5), hemorrhage (n=4), pneumonia (n=3), pneumonitis (n=2), cardiac arrest (n=2), sudden death (n=1), and cardiac failure (n=1).
Common adverse reactions (all grades) occurring in ≥20% of patients who received Retevmo in LIBRETTO-001, were edema (49%), diarrhea (47%), fatigue (46%), dry mouth (43%), hypertension (41%), abdominal pain (34%), rash (33%), constipation (33%), nausea (31%), headache (28%), cough (24%), vomiting (22%), dyspnea (22%), hemorrhage (22%), arthralgia (21%), and prolonged QT interval (21%).
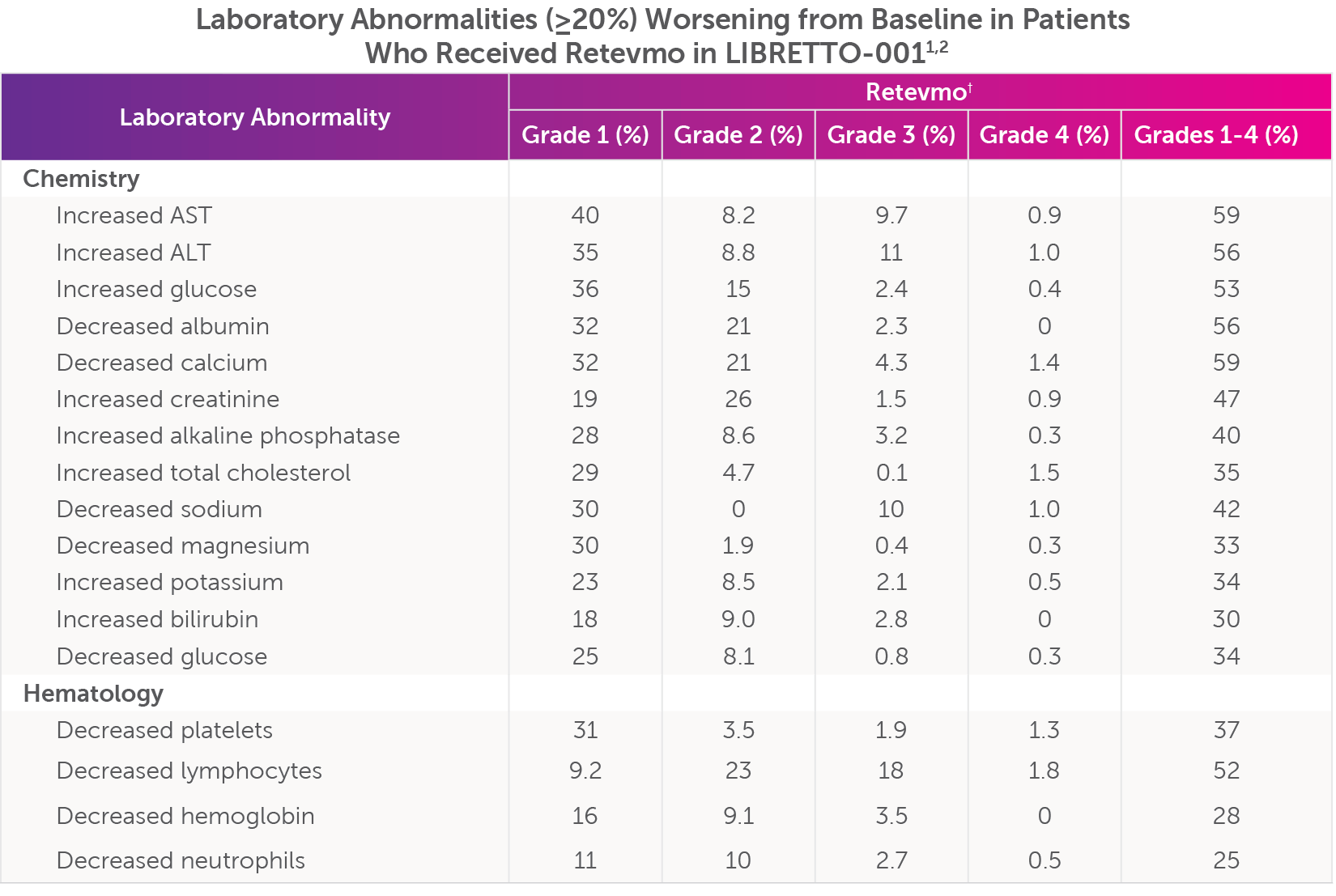
Laboratory abnormalities (all grades ≥20%; Grade 3-4) worsening from baseline in patients who received Retevmo in LIBRETTO-001, were increased AST (59%; 11%), decreased calcium (59%; 5.7%), increased ALT (56%; 12%), decreased albumin (56%; 2.3%), increased glucose (53%; 2.8%), decreased lymphocytes (52%; 20%), increased creatinine (47%; 2.4%), decreased sodium (42%; 11%), increased alkaline phosphatase (40%; 3.4%), decreased platelets (37%; 3.2%), increased total cholesterol (35%; 1.7%), increased potassium (34%; 2.7%), decreased glucose (34%; 1.0%), decreased magnesium (33%; 0.6%), increased bilirubin (30%; 2.8%), decreased hemoglobin (28%; 3.5%), and decreased neutrophils (25%; 3.2%).
Concomitant use of acid-reducing agents decreases selpercatinib plasma concentrations which may reduce Retevmo anti-tumor activity. Avoid concomitant use of proton-pump inhibitors (PPIs), histamine-2 (H2) receptor antagonists, and locally-acting antacids with Retevmo. If coadministration cannot be avoided, take Retevmo with food (with a PPI) or modify its administration time (with a H2 receptor antagonist or a locally-acting antacid).
Concomitant use of strong and moderate CYP3A inhibitors increases selpercatinib plasma concentrations which may increase the risk of Retevmo adverse reactions including QTc interval prolongation. Avoid concomitant use of strong and moderate CYP3A inhibitors with Retevmo. If concomitant use of a strong or moderate CYP3A inhibitor cannot be avoided, reduce the Retevmo dosage as recommended and monitor the QT interval with ECGs more frequently.
Concomitant use of strong and moderate CYP3A inducers decreases selpercatinib plasma concentrations which may reduce Retevmo anti-tumor activity. Avoid coadministration of Retevmo with strong and moderate CYP3A inducers.
Concomitant use of Retevmo with CYP2C8 and CYP3A substrates increases their plasma concentrations which may increase the risk of adverse reactions related to these substrates. Avoid coadministration of Retevmo with CYP2C8 and CYP3A substrates where minimal concentration changes may lead to increased adverse reactions. If coadministration cannot be avoided, follow recommendations for CYP2C8 and CYP3A substrates provided in their approved product labeling.
Retevmo is a P-glycoprotein (P-gp) inhibitor. Concomitant use of Retevmo with P-gp substrates increases their plasma concentrations, which may increase the risk of adverse reactions related to these substrates. Avoid coadministration of Retevmo with P-gp substrates where minimal concentration changes may lead to increased adverse reactions. If coadministration cannot be avoided, follow recommendations for P-gp substrates provided in their approved product labeling.
The safety and effectiveness of Retevmo have not been established in pediatric patients less than 12 years of age. The safety and effectiveness of Retevmo have been established in pediatric patients aged 12 years and older for medullary thyroid cancer (MTC) who require systemic therapy and for advanced RET fusion-positive thyroid cancer who require systemic therapy and are radioactive iodine-refractory (if radioactive iodine is appropriate). Use of Retevmo for these indications is supported by evidence from adequate and well-controlled studies in adults with additional pharmacokinetic and safety data in pediatric patients aged 12 years and older. Monitor open growth plates in adolescent patients. Consider interrupting or discontinuing Retevmo if abnormalities occur.
No dosage modification is recommended for patients with mild to severe renal impairment (estimated Glomerular Filtration Rate [eGFR] ≥15 to 89 mL/min, estimated by Modification of Diet in Renal Disease [MDRD] equation). A recommended dosage has not been established for patients with end-stage renal disease.
Reduce the dose when administering Retevmo to patients with severe hepatic impairment (total bilirubin greater than 3 to 10 times upper limit of normal [ULN] and any AST). No dosage modification is recommended for patients with mild or moderate hepatic impairment. Monitor for Retevmo-related adverse reactions in patients with hepatic impairment.
Please see full
Prescribing Information
for Retevmo.
SE HCP ISI All_21SEP22