LIBRETTO 531
L-531 Trial Design
LIBRETTO-531: A Phase III superiority trial evaluating Retevmo versus cabozantinib or vandetanib in RET-mutant advanced or metastatic MTC6-8
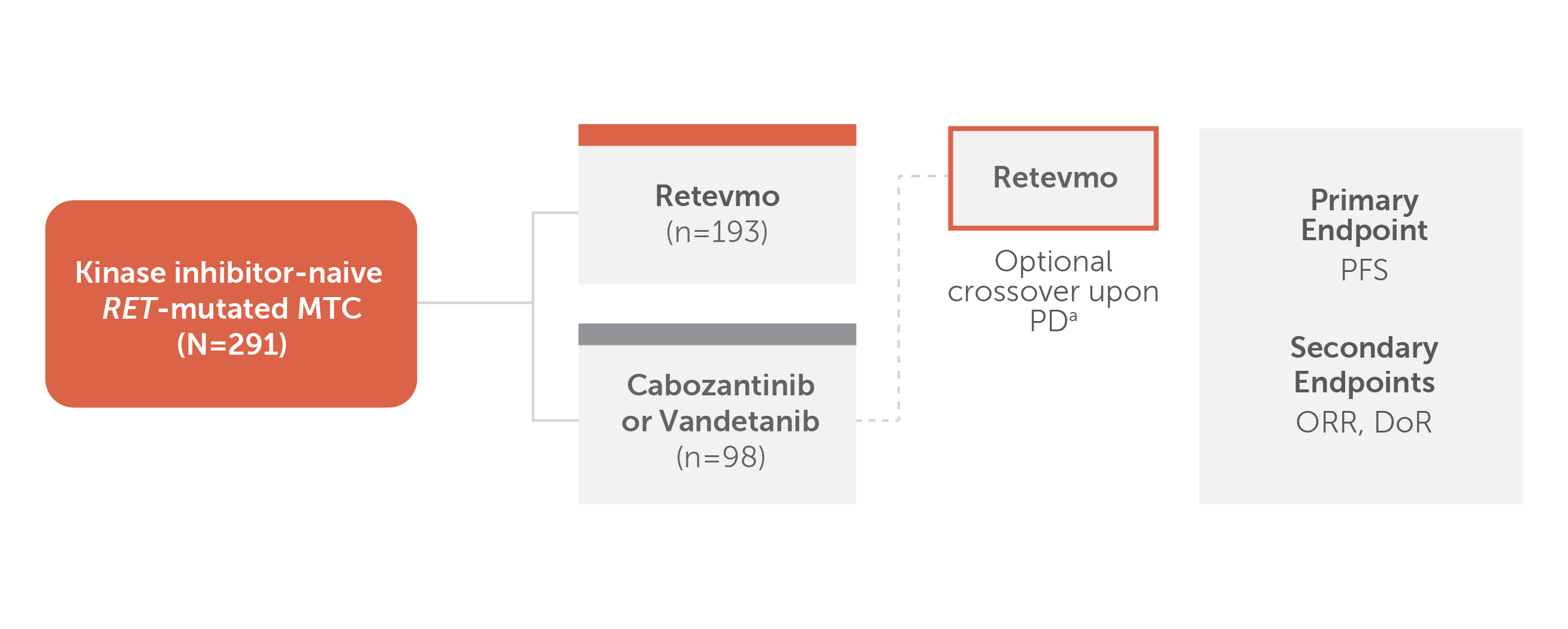
Chart showing trial design for LIBRETTO-531. Patients with kinase inhibitor-naive RET-mutant MTC (N=291) were randomized 2:1. One hundred ninety-three patients were randomized to Retevmo; 98 patients were randomized to cabozantinib or vandetanib. (73 patients and 25 patients received cabozantinib and vandetanib, respectively, within the control arm. Stratification factors: investigator’s choice of treatment if randomized to control arm [choice/intent of treatment regimen must be declared prior to randomization], mutation status [M918T versus other].) Crossover to Retevmo was optional for patients in the control arm upon confirmed progression by IRC. The primary endpoint was PFS. Secondary endpoints included ORR and DoR. (All primary and secondary endpoints listed were reviewed by IRC per RECIST v1.1.)
a PD was confirmed by IRC
Trial excluded patients who had presence of other validated oncogenic drivers. Retevmo was dosed at 160 mg BID. Cabozantinib was dosed at 140 mg QD and vandetanib was dosed at 300 mg QD.
BID=twice a day; DoR=duration of response; IRC=independent review committee; MTC=medullary thyroid cancer; ORR=overall response rate; PD=progressive disease; PFS=progression-free survival; QD=every day; RECIST=Response Evaluation Criteria in Solid Tumours; RET=rearranged during transfection
PFS
In patients with RET-mutant advanced or metastatic MTC
Retevmo demonstrated superior PFS compared to cabozantinib or vandetanib8,9
Median PFS: Not yet reached (95% CI: NE, NE) with Retevmo (n=193) vs 16.8 months (95% CI: 12.2, 25.1) with cabozantinib or vandetanib (n=98)8,9,‖
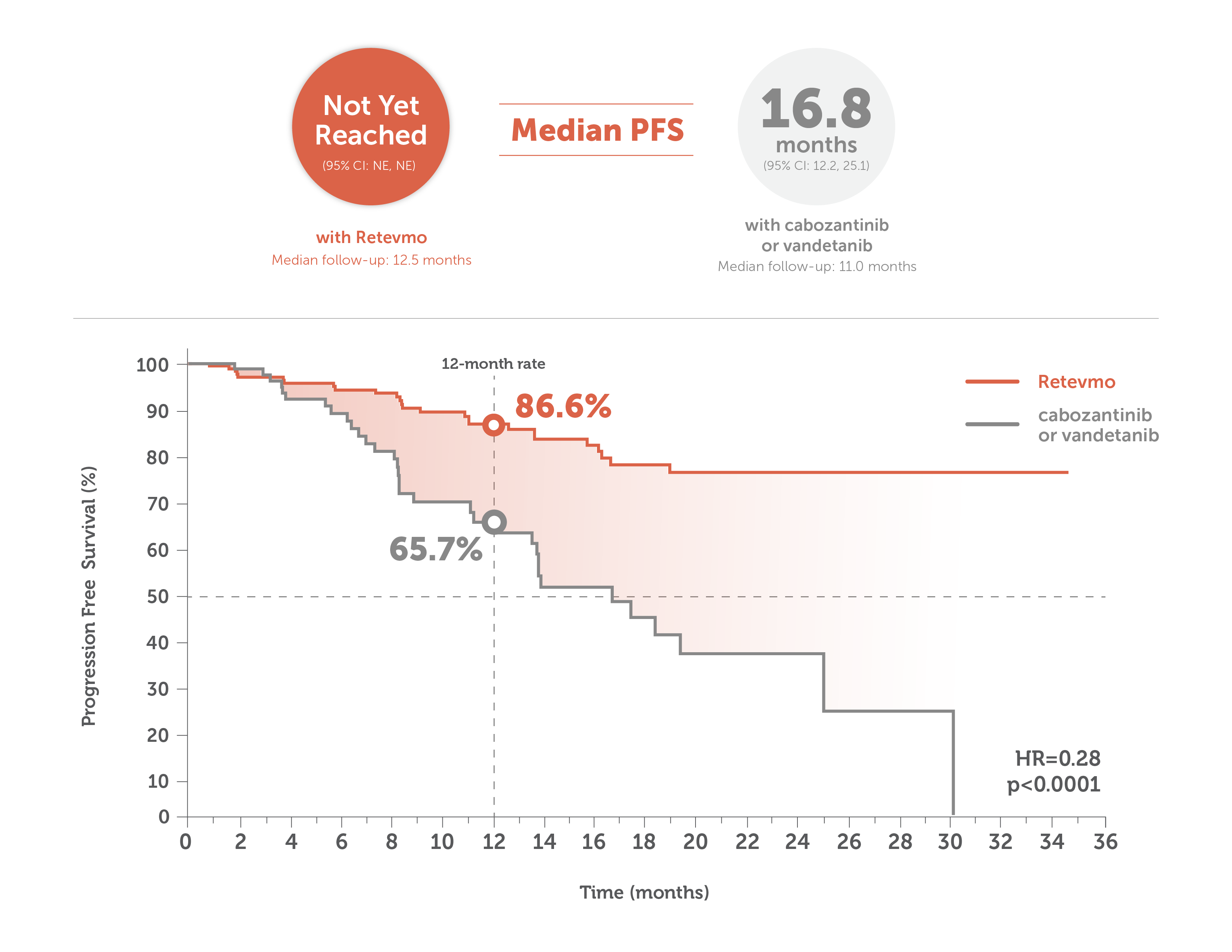
This image shows median PFS in Retevmo-treated patients (n=193) versus those treated with cabozantinib or vandetanib (n=98). PFS was assessed in all randomized patients and was based on an interim analysis with a data cutoff date of May 22, 2023. For patients treated with Retevmo, median PFS was not yet reached (95% CI not estimable) with median follow-up of 12.5 months. In patients treated with cabozantinib or vandetanib, median PFS was 16.8 months (95% CI: 12.2, 25.1) with median follow-up of 11.0 months. An accompanying Kaplan-Meier curve shows progression-free survival for Retevmo-treated patients of 86.6% at 12 months. PFS for patients treated with cabozantinib or vandetanib was 65.7% at 12 months. Hazard ratio was 0.28 (p<0.0001).
PFS was assessed in all randomized patients. Based on an interim analysis with a data cutoff date of May 22, 2023.8,9
‖ Median follow-up time for patients treated with Retevmo was 12.5 months. Median follow-up time for patients in the control arm was 11.0 months.
HR=hazard ratio; MTC=medullary thyroid cancer; NE=not estimable; PFS=progression-free survival; RET=rearranged during transfection
Select Important Safety Information
Hepatotoxicity: Serious hepatic adverse reactions occurred in 3% of patients treated with Retevmo. Increased aspartate aminotransferase (AST) occurred in 59% of patients, including Grade 3 or 4 events in 11% and increased alanine aminotransferase (ALT) occurred in 55% of patients, including Grade 3 or 4 events in 12%. Monitor ALT and AST prior to initiating Retevmo, every 2 weeks during the first 3 months, then monthly thereafter and as clinically indicated. Withhold, reduce dose, or permanently discontinue Retevmo based on the severity.
ORR and DoR
Overall response rate and duration of response for Retevmo vs cabozantinib or vandetanib8,9,¶
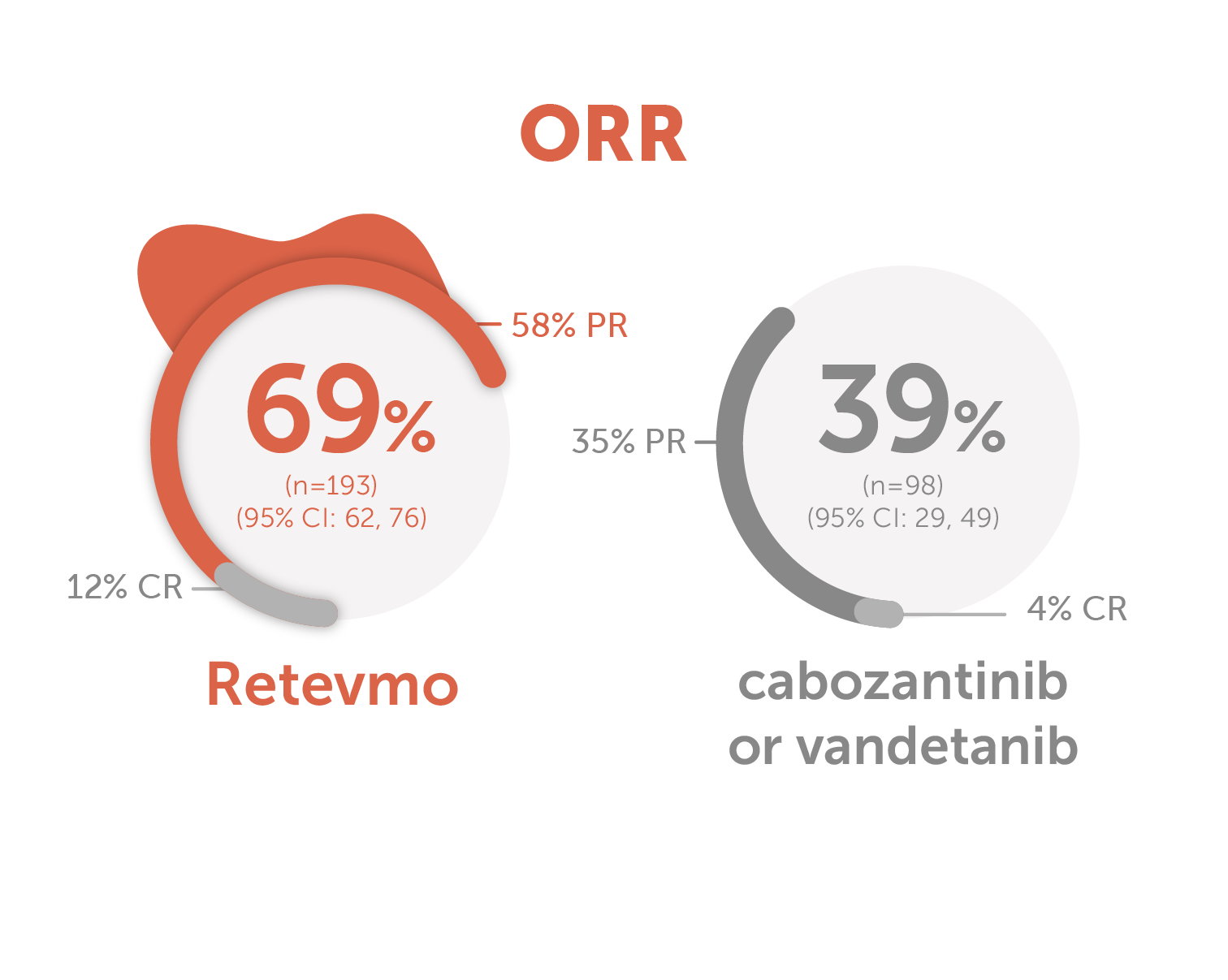
Image showing Overall Response Rate for Retevmo versus cabozantinib or vandetanib. ORR for Retevmo (n=193) was 69% (95% CI: 62, 76). Within this group, 58% of patients showed Partial Response and 12% showed Complete Response. In patients treated with cabozantinib or vandetanib (n=98), ORR was 39% (95% CI: 29, 49). Within this group, 35% of patients showed Partial Response and 4% showed Complete Response.
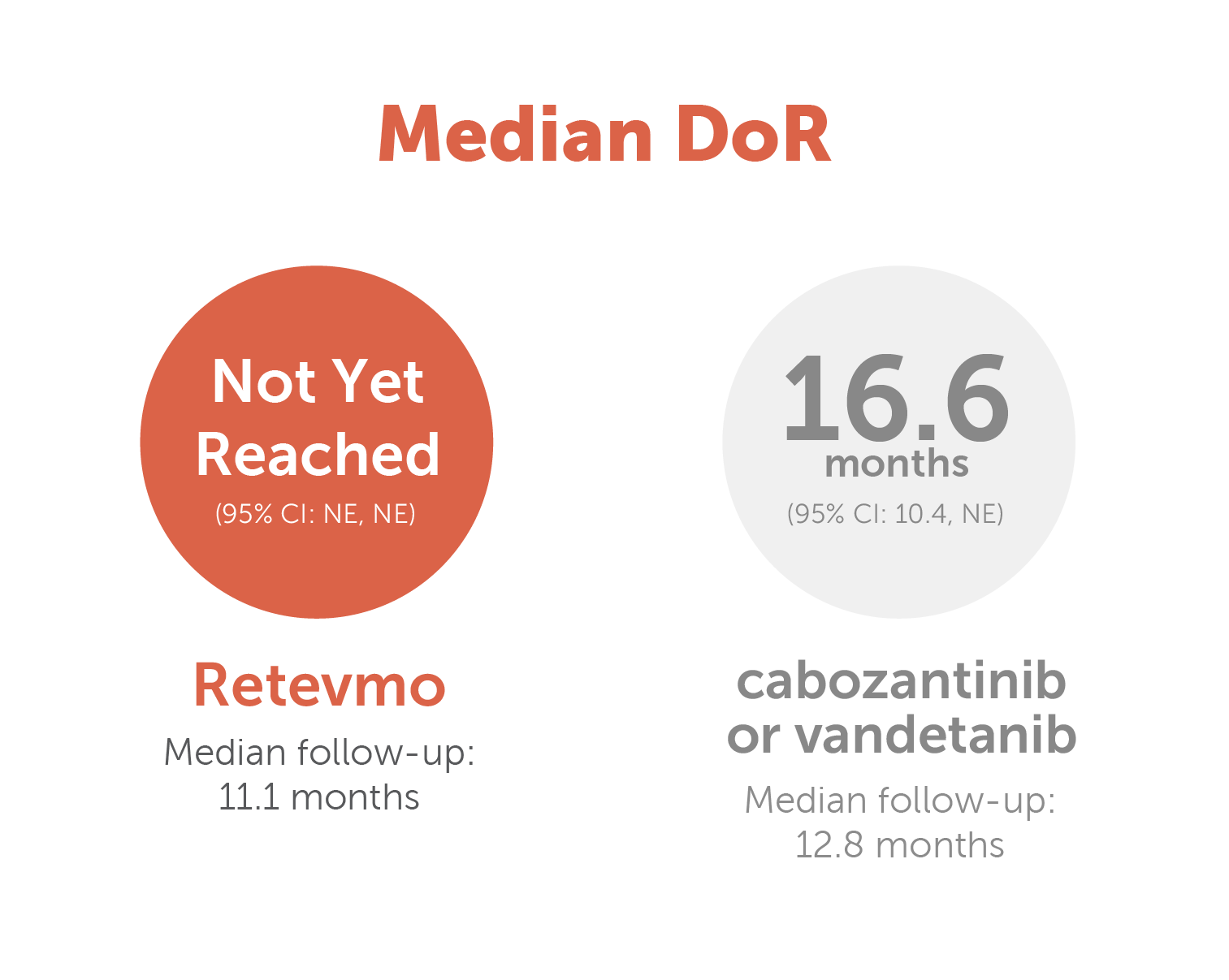
Image showing Duration of Response for Retevmo versus cabozantinib or vandetanib. DoR for Retevmo was not yet reached (95% CI: NE, NE). Median follow-up was 11.1 months. In patients treated with cabozantinib or vandetanib DoR was 16.6 months (95% CI: 10.4, NE). Median follow-up was 12.8 months.
- ORR, a secondary endpoint, was defined as CR+PR and was assessed by the IRC according to RECIST v1.1.
- Based on an interim analysis with a data cutoff date of May 22, 2023.8,9
¶ Due to rounding, percentages presented may not add up to the indicated totals.
** Median duration of response follow-up for patients treated with Retevmo was 11.1 months. Median follow-up time for patients treated with cabozantinib or vandetanib was 12.8 months.
CI=confidence interval; CR=complete response; DoR=duration of response; IRC=independent review committee; MTC=medullary thyroid cancer; NE=not estimable; NSCLC=non-small cell lung cancer; ORR=overall response rate; PR=partial response; RECIST= Response Evaluation Criteria in Solid Tumours; RET=rearranged during transfection
Safety
Safety and tolerability were evaluated for Retevmo in LIBRETTO-5318,10
Select Adverse Events (≥20%) in Patients Treated with Retevmo in LIBRETTO-531
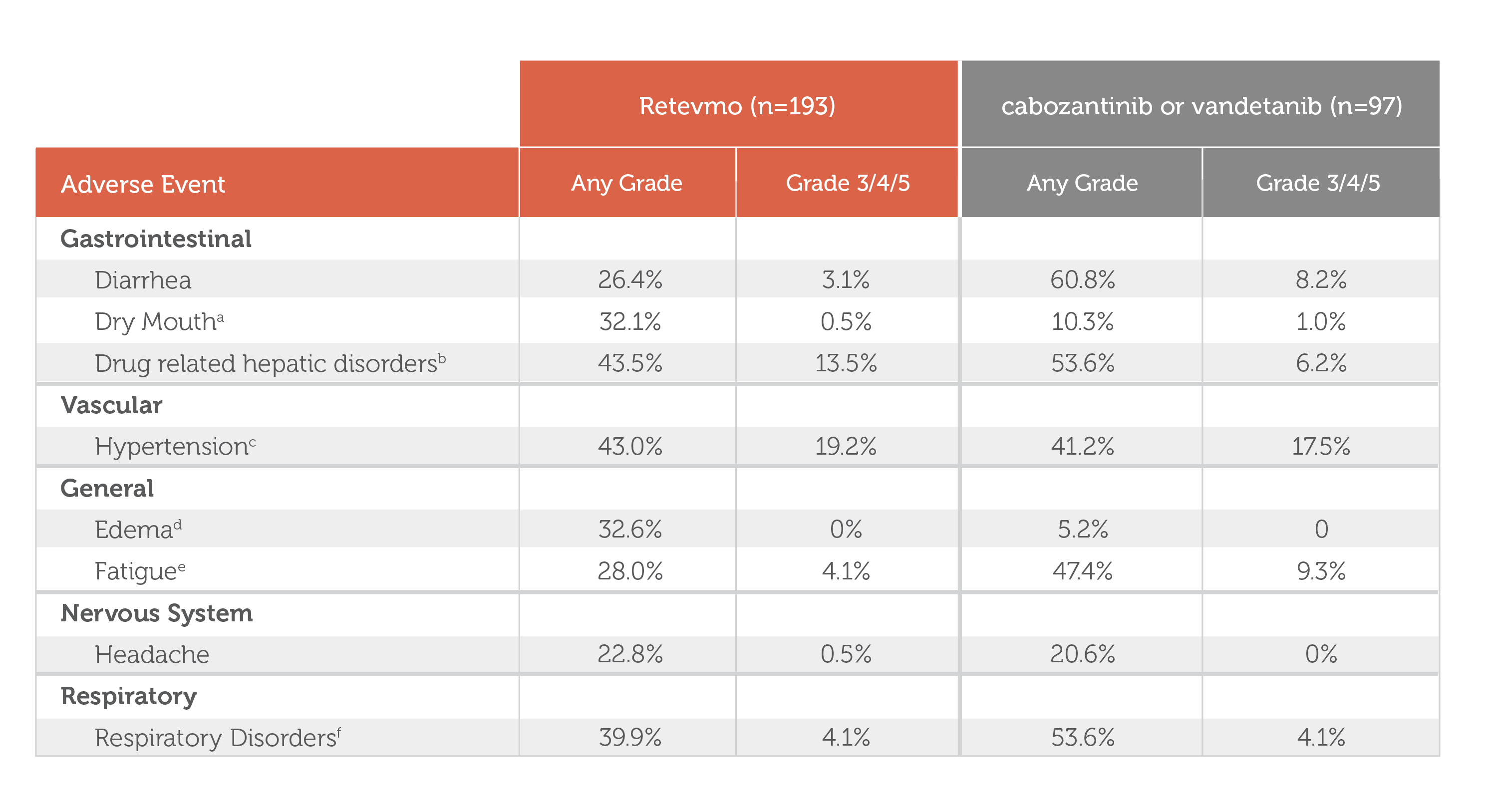
- Retevmo, Any Grade: In Retevmo-treated patients (n=193), gastrointestinal adverse events of any Grade included diarrhea (26.4%), dry mouth (32.1%), and drug related hepatic disorders (43.5%). Vascular adverse events of any Grade included hypertension (43.0%). General adverse events of any Grade included edema (32.6%) and fatigue (28.0%). Nervous System adverse events of any Grade included headache (22.8%). Respiratory adverse events of any Grade included respiratory disorders (39.9%).
- Retevmo, Grade 3/4/5: In Retevmo-treated patients (n=193), gastrointestinal adverse events of Grade 3/4/5 included diarrhea (3.1%), dry mouth (0.5%), and drug related hepatic disorders (13.5%). Vascular adverse events of Grade 3/4/5 included hypertension (19.2%). General adverse events of Grade 3/4/5 included fatigue (4.1%). Nervous System adverse events of Grade 3/4/5 included headache (0.5%). Respiratory adverse events of Grade 3/4/5 included respiratory disorders (4.1%).
- Cabozantinib or Vandetanib, Any Grade: In cabozantinib- or vandetanib-treated patients (n=97), gastrointestinal adverse events of any Grade included diarrhea (60.8%), dry mouth (10.3%), and drug related hepatic disorders (53.6%). Vascular adverse events of any Grade included hypertension (41.2%). General adverse events of any Grade included edema (5.2%) and fatigue (47.4%). Nervous System adverse events of any Grade included headache (20.6%). Respiratory adverse events of any Grade included respiratory disorders (53.6%).
- Cabozantinib or Vandetanib, Grade 3/4/5: In cabozantinib- or vandetanib-treated patients (n=97), gastrointestinal adverse events of Grade 3/4/5 included diarrhea (8.2%), dry mouth (1.0%), and drug related hepatic disorders (6.2%). Vascular adverse events of Grade 3/4/5 included hypertension (17.5%). General adverse events of Grade 3/4/5 included fatigue (9.3%). Respiratory adverse events of Grade 3/4/5 included respiratory disorders (4.1%).
a Dry mouth includes dry mouth, mucosal dryness
b Drug related hepatic disorders include alanine aminotransferase increased, aspartate aminotransferase increased, blood alkaline phosphatase increased, blood bilirubin increased, ascites, GGT increased, hyperbilirubinemia, hepatic function abnormal, hypoalbuminemia, bilirubin conjugate increased, hepatic enzyme increased, blood bilirubin unconjugated increased, liver injury, cholestasis, drug-induced liver injury, hepatic steatosis, liver function test increased, transaminases increased, bilirubin urine present, GGT abnormal, glutamate dehydrogenase increased, hepatic lesion, hepatic pain, hepatotoxicity, INR increased, jaundice LFT abnormal
c Hypertension includes hypertension, blood pressure increased
d Edema includes peripheral edema, face edema, edema, periorbital edema, edema, eyelid edema, localized edema, swelling face, swelling of eyelid, periorbital edema, peripheral swelling, eye swelling, generalized edema
e Fatigue includes fatigue, asthenia, malaise
f Respiratory disorders includes COVID-19, cough, dyspnea, epistaxis, oropharyngeal pain, upper respiratory tract infection, dysphonia, nasal dryness, sinusitis, chest pain, nasopharyngitis, pleural effusion, influenza, musculoskeletal chest pain, rhinitis, bronchitis, pharyngitis, pneumonia, productive cough, pulmonary embolism, viral upper respiratory tract infection, lower respiratory tract infection, nasal obstruction, non-cardiac chest pain, allergic cough, aspiration, asthma, bronchitis chronic, bronchostenosis, COVID-19 pneumonia, chest discomfort, chronic obstructive pulmonary disease, dyspnea exertional, hemoptysis, hiatus hernia, hiccups, increased upper airway secretion, interstitial lung disease, lung disorder, nasal discomfort, nasal inflammation, nasal injury, nasal ulcer, oropharyngeal blistering, oropharyngeal discomfort, pharyngeal inflammation, pleurisy, pneumonitis, pulmonary edema, respiratory tract infection, rhinitis allergic, tracheal scarring, tracheostomy malfunction, vocal cord paresis
Select Laboratory Abnormalities (≥20%) Worsening from Baseline in Patients Treated with Retevmo in LIBRETTO-531
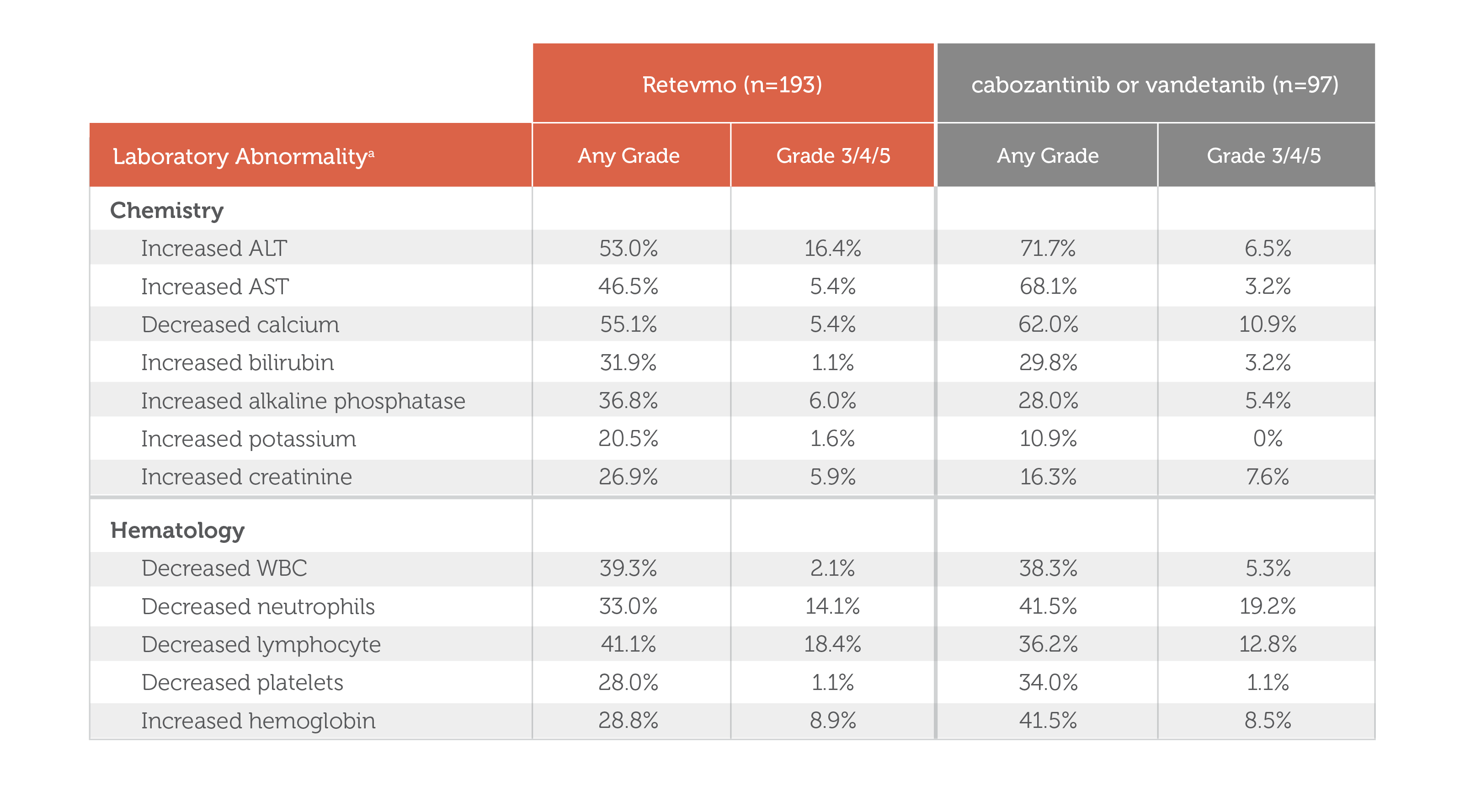
- Retevmo, Any Grade: In Retevmo-treated patients (n=193), chemistry-related laboratory abnormalities of any Grade included increased ALT (53.0%), increased AST (46.5%), decreased calcium (55.1%), increased bilirubin (31.9%), increased alkaline phosphatase (36.8%), increased potassium (20.5%), and increased creatinine (26.9%). Hematology-related laboratory abnormalities of any Grade included decreased WBC (39.3%), decreased neutrophils (33.0%), decreased lymphocyte (41.1%), decreased platelets (28.0%), and increased hemoglobin (28.8%).
- Retevmo, Grade 3/4/5: In Retevmo-treated patients (n=193), chemistry-related laboratory abnormalities of Grade 3/4/5 included increased ALT (16.4%), increased AST (5.4%), decreased calcium (5.4%), increased bilirubin (1.1%), increased alkaline phosphatase (6.0%), increased potassium (1.6%), and increased creatinine (5.9%). Hematology-related laboratory abnormalities of Grade 3/4/5 included decreased WBC (2.1%), decreased neutrophils (14.1%), decreased lymphocyte (18.4%), decreased platelets (1.1%), and increased hemoglobin (8.9%).
- Cabozantinib or vandetanib, Any Grade: In cabozantinib- or vandetanib-treated patients (n=97), chemistry-related laboratory abnormalities of any Grade included increased ALT (71.7%), increased AST (68.1%), decreased calcium (62.0%), increased bilirubin (29.8%), increased alkaline phosphatase (28.0%), increased potassium (10.9%), and increased creatinine (16.3%). Hematology-related laboratory abnormalities of any Grade included decreased WBC (38.3%), decreased neutrophils (41.5%), decreased lymphocyte (36.2%), decreased platelets (34.0%), and increased hemoglobin (41.5%).
- Cabozantinib or vandetanib, Grade 3/4/5: In cabozantinib- or vandetanib-treated patients (n=97), chemistry-related laboratory abnormalities of Grade 3/4/5 included increased ALT (6.5%), increased AST (3.2%), decreased calcium (10.9%), increased bilirubin (3.2%), increased alkaline phosphatase (5.4%), and increased creatinine (7.6%). Hematology-related laboratory abnormalities of Grade 3/4/5 included decreased WBC (5.3%), decreased neutrophils (19.2%), decreased lymphocyte (12.8%), decreased platelets (1.1%), and increased hemoglobin (8.5%).
a Denominator for each laboratory parameter is based on the number of patients with a baseline and post-treatment laboratory value available, which ranged from 185 to 191 patients for Retevmo and 92 to 94 patients for the control arm.8,10
- 4.7% (n=9) of patients permanently discontinued Retevmo (n=193) due to adverse events. 2.1% (n=4) were considered treatment-related, as assessed by trial investigator. Adverse events resulting in permanent discontinuation in ≥0.5% of patients included multiple organ dysfunction (n=1), edema peripheral (n=1), sudden death (n=1), aspartate aminotransferase increased (n=1), diabetic ketoacidosis (n=1), chronic kidney disease (n=1), retinopathy (n=1), COVID-19 (n=1), and somatic symptom disorder (n=1).
- Serious treatment emergent adverse events occurred in 21.8% (n=42) of patients who received Retevmo, of which 5.2% (n=10) were considered related to study drug. The most frequently reported serious treatment emergent adverse events (in ≥1% of patients) were hypertension (n=2), pneumonia (n=3), pyrexia (n=3), urinary tract infection (n=2), colporrhaphy (n=1), and ovarian cyst (n=1).
- Fatal adverse events occurred in 2.1% (n=4) of patients in the Retevmo arm while on treatment. The causes of death included COVID-19 (n=1), diabetic ketoacidosis (n=1), multiple organ dysfunction (n=1), sudden death (n=1). None of the deaths were considered related to study treatment.
- Dose interruptions and dose reductions due to AEs occurred in 56% (n=108) and 38.9% (n=75) of patients who received Retevmo, respectively. See full Prescribing Information for dose modifications.
- AEs requiring dosage interruption in ≥2% of patients included increased ALT, increased AST, COVID-19, diarrhea, electrocardiogram QT prolongation, hypertension, pyrexia, scheduling conflicts, and other epidemic/pandemic reasons.
- AEs requiring dosage reductions in ≥2% of patients included increased ALT, increased AST, electrocardiogram QT prolongation, fatigue, hypertension, and rash.
- Clinically relevant toxicities included decreased appetite (11.9%), chylothorax (6.7%), hemorrhages (6.7%), interstitial lung disease/pneumonitis (1.0%), and hypersensitivity (0.5%). No reports of hypothyroidism.
- Grade according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 5.0
AE=adverse event; ALT=alanine transaminase; AST=aspartate aminotransferase; COVID-19=coronavirus disease 2019; GGT=gamma-glutamyl transferase; INR=international normalized ratio; LFT=liver function test; WBC=white blood cell
Select Important Safety Information
Severe, life-threatening, and fatal interstitial lung disease (ILD)/pneumonitis can occur in patients treated with Retevmo. ILD/pneumonitis occurred in 1.8% of patients who received Retevmo, including 0.3% with Grade 3 or 4 events, and 0.3% with fatal reactions. Monitor for pulmonary symptoms indicative of ILD/pneumonitis. Withhold Retevmo and promptly investigate for ILD in any patient who presents with acute or worsening of respiratory symptoms which may be indicative of ILD (e.g., dyspnea, cough, and fever). Withhold, reduce dose, or permanently discontinue Retevmo based on severity of confirmed ILD.
L-001 Efficacy Summary
In patients with RET-mutant advanced or metastatic MTC
Retevmo was granted approval based on LIBRETTO-001, a Phase I/II trial
Major efficacy outcome measures for patients previously treated* with cabozantinib and/or vandetanib (n=55)1:
- ORR = 69% (95% CI: 55, 81)
- Median DoR = Not yet reached (95% CI: 19.1, NE); Median follow-up: 14.1 months2
Efficacy in cabozantinib/vandetanib-naive patients (n=88)1

Graphic showing Overall Response Rate and Median Duration of Response for Retevmo treatment in cabozantinib/vandetanib-naive patients. ORR was 73% (95% CI: 62,82), with 11% complete response (CR) and 61% PR (partial response). Median DoR was 22.0 months (95% CI not estimable). Median follow-up was 7.8 months.
- Time-to-event endpoints are not interpretable in a single-arm study. The clinical significance of this descriptive analysis is not known.
- ORR was defined as CR + PR and was assessed by IRC according to RECIST v1.1. All results reviewed by an IRC.1
*The efficacy of Retevmo was evaluated in 55 patients with RET-mutant advanced MTC who were previously treated with cabozantinib or vandetanib enrolled into a cohort of LIBRETTO-001.1
CI=confidence interval; CR=complete response; DoR=duration of response; IRC=independent review committee; MTC=medullary thyroid cancer; NE=not estimable; ORR=overall response rate; PR=partial response; RECIST=Response Evaluation Criteria in Solid Tumours; RET=rearranged during transfection
L-001 Trial Design
The phase I/II, multicohort, open-label, single-arm, multicenter LIBRETTO-001 trial included a pooled safety population of 796 patients with locally advanced or metastatic RET fusion-positive NSCLC† (n=356), advanced or metastatic RET fusion-positive thyroid cancer (non-MTC)‡ (n=54), advanced or metastatic RET-mutant MTC (n=319), and other cancers, including patients with RET alterations in other tumors (n=67§). Major efficacy outcomes were ORR and DoR, and were evaluated in 527 patients. Other efficacy outcomes, evaluated in subsets of patients, included CNS ORR, CNS DoR, PFS, OS, time to response, and best change in tumor size from baseline. In phase II, the dose for Retevmo was 160 mg PO BID.1,3-5
†Patients with locally advanced or metastatic RET fusion-positive NSCLC who had progressed on platinum-based chemotherapy and those without prior systemic therapy were enrolled in separate cohorts.1
‡Non-MTC by histology included papillary (n=21), poorly differentiated (n=3), anaplastic (n=2), and Hurthle cell (n=1).1
§Other tumors included pancreatic adenocarcinoma (n=11), colorectal cancer (n=10), and salivary cancer (n=4).1
BID=twice daily; CNS=central nervous system; DoR=duration of response; NSCLC=non-small cell lung cancer; ORR=objective response rate; OS=overall survival; PFS=progression-free survival; PO=orally; RET=rearranged during transfection.
Select Important Safety Information
Hypertension occurred in 41% of patients, including Grade 3 hypertension in 20% and Grade 4 in one (0.1%) patient. Overall, 6.3% had their dose interrupted and 1.3% had their dose reduced for hypertension. Treatment-emergent hypertension was most commonly managed with anti-hypertension medications. Do not initiate Retevmo in patients with uncontrolled hypertension. Optimize blood pressure prior to initiating Retevmo. Monitor blood pressure after 1 week, at least monthly thereafter, and as clinically indicated. Initiate or adjust anti-hypertensive therapy as appropriate. Withhold, reduce dose, or permanently discontinue Retevmo based on the severity.
References: 1. Retevmo (selpercatinib). Prescribing Information. Lilly USA, LLC. 2. Wirth LJ, Sherman E, Robinson B, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med. 2020;383:825-835. 3. Phase 1/2 study of LOXO-292 in patients with advanced solid tumors, RET fusion-positive solid tumors, and medullary thyroid cancer (LIBRETTO-001). https://clinicaltrials.gov/ct2/show/NCT03157128. Updated June 9, 2022. Accessed June 14, 2022. 4. Data on File, Lilly USA, LLC, DOF-SE-US-0063. 5. Drilon A, Subbiah V, Gautschi O, et al. Durability of efficacy and safety with selpercatinib in patients with RET fusion+ non-small-cell lung cancer: LIBRETTO-001. Poster presented at: European Lung Cancer Congress; March 30–April 2, 2022. Poster 27P. 6. Wirth L, Borse M, Elisei R, et al. LIBRETTO-531: a phase III study of selpercatinib in multikinase inhibitor-naïve RET-mutant medullary thyroid cancer. Future Oncol. 2022;18(28):3143-3150. doi:10.2217/fon-2022-0657. 7. Data on File. Lilly USA, LLC. DOF-SE-US-0084. 8. Hadoux J, Elisei R, Brose MS, et al. Phase 3 trial of selpercatinib in RET-mutant MKI-naïve advanced MTC. N Engl J Med. In press. 9. Data on File. Lilly USA, LLC. DOF-SE-US-0081. 10. Data on File. Lilly USA, LLC. DOF-SE-US-0083.
INDICATIONS
Retevmo is a kinase inhibitor indicated for the treatment of:
- adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with a rearranged during transfection (RET) gene fusion, as detected by an FDA-approved test
- adult and pediatric patients 12 years of age and older with advanced or metastatic medullary thyroid cancer (MTC) with a RET mutation, as detected by an FDA-approved test, who require systemic therapy*
- adult and pediatric patients 12 years of age and older with advanced or metastatic thyroid cancer with a RET gene fusion, as detected by an FDA-approved test, who require systemic therapy and who are radioactive iodine-refractory (if radioactive iodine is appropriate)*
- adult patients with locally advanced or metastatic solid tumors with a RET gene fusion that have progressed on or following prior systemic treatment or who have no satisfactory alternative treatment options*
*These indications are approved under accelerated approval based on overall response rate (ORR) and duration of response (DoR). Continued approval for these indications may be contingent upon verification and description of clinical benefit in confirmatory trials.