Retevmo was evaluated in a phase I/II trial: the largest trial ever reported in patients with RET-driven cancer1-2
796 patients with RET-altered advanced or metastatic solid tumors were included in LIBRETTO-001, an open-label, single-arm, multicenter, phase I/II, multicohort trial.1-5
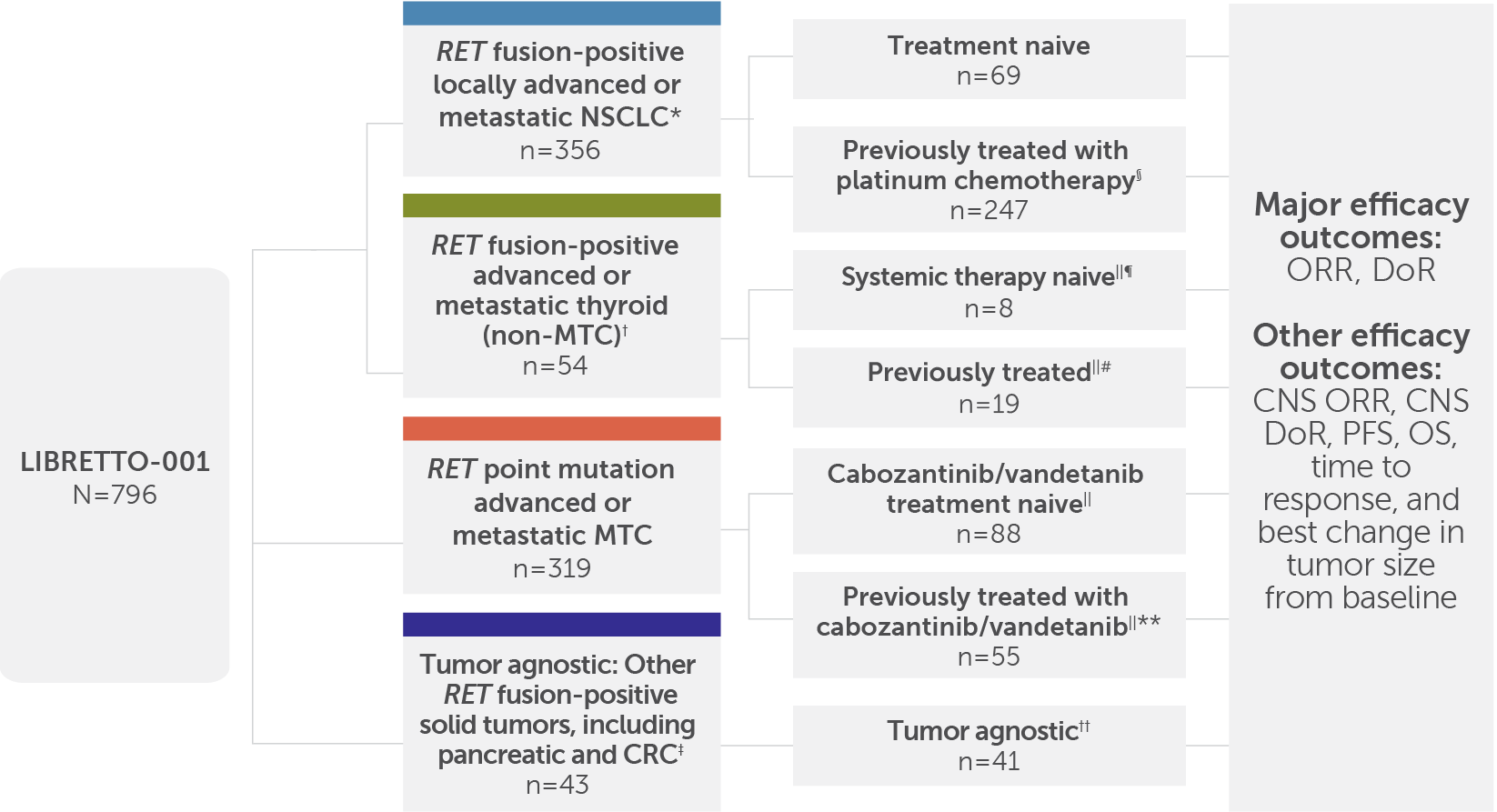
LIBRETTO-001 included 796 patients, and the major efficacy outcomes were ORR and DoR; other efficacy outcomes included CNS ORR, CNS DoR, PFS, OS, time to response, and best change in tumor size from baseline.1,3,5
The overall safety analysis included 24 patients with other cancers, including cancers without a RET alteration.4
Phase 1 dose escalation: Retevmo dosed at 20 mg QD–240 mg BID. Intra-patient dose escalation was allowed by protocol.3
Phase 2 dose: Retevmo dosed at 160 mg BID.3
See full Prescribing Information for dosing instructions.
Objective response rate (ORR) was defined as complete response (CR) + partial response (PR) and was assessed by independent review committee (IRC) according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1.1
The intent-to-treat (ITT) population was analyzed in the LIBRETTO-001 trial because this type of analysis avoids the selective exclusion of patients, which can lead to a biased assessment of an intervention's effectiveness.6-9
*Patients with advanced or metastatic RET fusion-positive NSCLC who had progressed on platinum-based chemotherapy and those without prior systemic therapy were enrolled in separate cohorts.1
†Non-MTC by histology included papillary (n=21), poorly differentiated (n=3), anaplastic (n=2), and Hurthle cell (n=1).1
‡Efficacy was evaluated in 41 patients, including those with the following tumor types: pancreatic adenocarcinoma (n=11); colorectal (n=10); salivary (n=4); unknown primary (n=3); breast (n=2); sarcoma (soft tissue) (n=2); xanthogranuloma (n=2); carcinoid (bronchial) (n=1); carcinoma of the skin (n=1), cholangiocarcinoma (n=1); ovarian (n=1); pulmonary carcinosarcoma (n=1); rectal neuroendocrine (n=1); small intestine (n=1).1
§Efficacy was evaluated in 247 adult patients with advanced or metastatic RET fusion-positive NSCLC who were previously treated with platinum chemotherapy enrolled into a cohort of LIBRETTO-001. All 247 patients received systemic therapy (with a median of 2 prior systemic regimens). 144 of the 247 patients received prior anti-PD-1/PD-L1 therapy, and 85 of the 247 patients received a prior MKI.3
ǁNumber of patients included in the initial efficacy analysis. Efficacy was based on patients who had at least 6 months of follow-up.4
¶Patients in this cohort received no prior systemic therapy other than radioactive iodine (RAI).1
#Patients in this cohort received a prior systemic therapy (including sorafenib, lenvatinib, or both) other than RAI.1
**The efficacy of Retevmo was evaluated in 55 patients with RET-mutant advanced MTC who were previously treated with cabozantinib or vandetanib enrolled into a cohort of LIBRETTO-001.1
††Patients in this cohort could have received prior systemic therapies with a median of 2 prior systemic therapies (range 0-9).1
BID=twice daily; CNS=central nervous system; CRC=colorectal cancer; DoR=duration of response; MKI=multikinase inhibitor; MTC=medullary thyroid cancer; NSCLC=non-small cell lung cancer; OS=overall survival; PD-1=programmed cell death 1; PD-L1=programmed death-ligand 1; PFS=progression-free survival; QD=once daily; RET=rearranged during transfection.
See efficacy results in patients with certain RET-driven cancers
Response in patients with locally advanced or metastatic RET fusion-positive NSCLC1
Treatment-naive patients (n=69)1,10
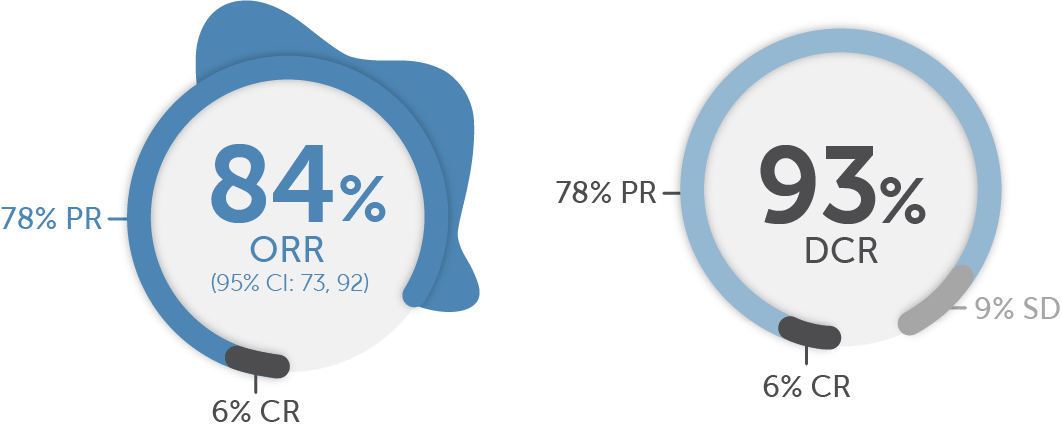
Objective response rate in treatment-naive patients in LIBRETTO-001 was 84% (95% CI: 73, 92; 6% CR + 78% PR).1
Median DoR was 20.2 months in treatment-naive patients in LIBRETTO-001 (95% CI: 13, NE). The median follow-up was 20.3 months.1,2
A 93% DCR (6% CR + 78% PR + 9% SD) was observed in treatment-naive patients1
Median DoR
20.2 months
(95% CI: 13, NE)
Median follow-up: 20.3 months1,3
The major efficacy outcome measures in LIBRETTO-001 were objective response rate (ORR) and duration of response (DoR). Disease control rate (DCR) was not a prespecified endpoint and is a post-hoc calculation. DCR is defined as ORR (CR + PR) + SD.
Stable disease (SD) is defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for progressive disease (PD), per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. In the setting of a single-arm trial without the ability to compare with a control arm provided by a randomized trial, the interpretation and clinical relevance of a best overall response of SD are not clear, and it is not possible to determine if SD is a result of natural disease progression or treatment with Retevmo.1,11
For treatment-naive patients who responded (n=58) to treatment, the median time to objective response was 1.8 months (range: 0.7, 10.8)12‡‡
Time to any and best response was a prespecified secondary endpoint.13
Time-to-event endpoints are not interpretable in a single-arm study.
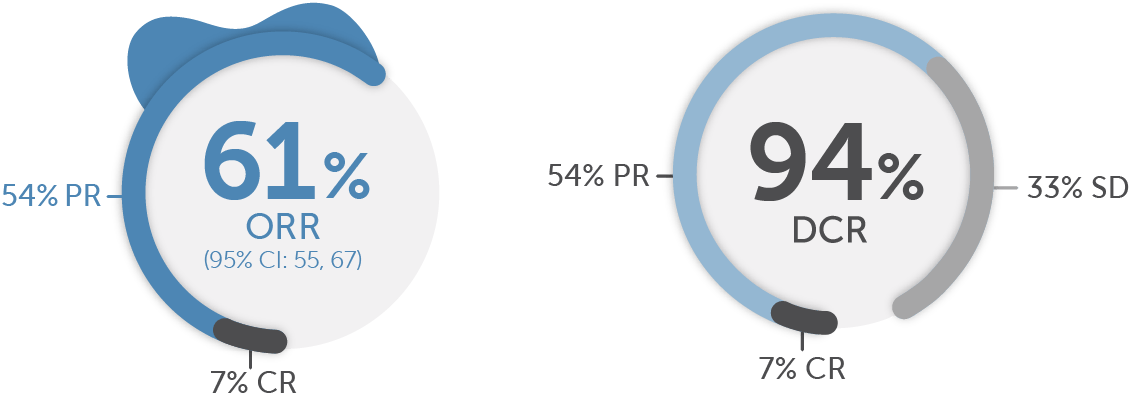
Previously treated§§ patients (n=247)1,10
Median DoR
28.6 months
(95% CI: 20, NE)
Median follow-up: 21.2 months1,3
For previously treated§§ patients who responded (n=151) to treatment, the median time to objective response was 1.9 months (range: 0.7, 21.9)12‡‡
Time to any and best response was a prespecified secondary endpoint.13
Time-to-event endpoints are not interpretable in a single-arm study.
All results reviewed by an IRC.1,9,12,13
Due to rounding, numbers presented may not add up to the totals indicated and percentages may not reflect the absolute figures for the same reason.
‡‡According to the protocol, scans were to be performed every 8 weeks (±7 days). The protocol allowed investigators to conduct an initial scan at 4 weeks (±7 days).14
§§Efficacy was evaluated in 247 adult patients with locally advanced or metastatic RET fusion-positive NSCLC who were previously treated with platinum chemotherapy enrolled into a cohort of LIBRETTO-001. All 247 patients received systemic therapy (with a median of 2 prior systemic regimens).1
Retevmo had CNS activity in patients with measurable brain metastases. CNS ORR was observed in the LIBRETTO-001 trial1
Of the 21 patients with measurable disease, 3 patients received RT to the brain within 2 months prior to study entry.1
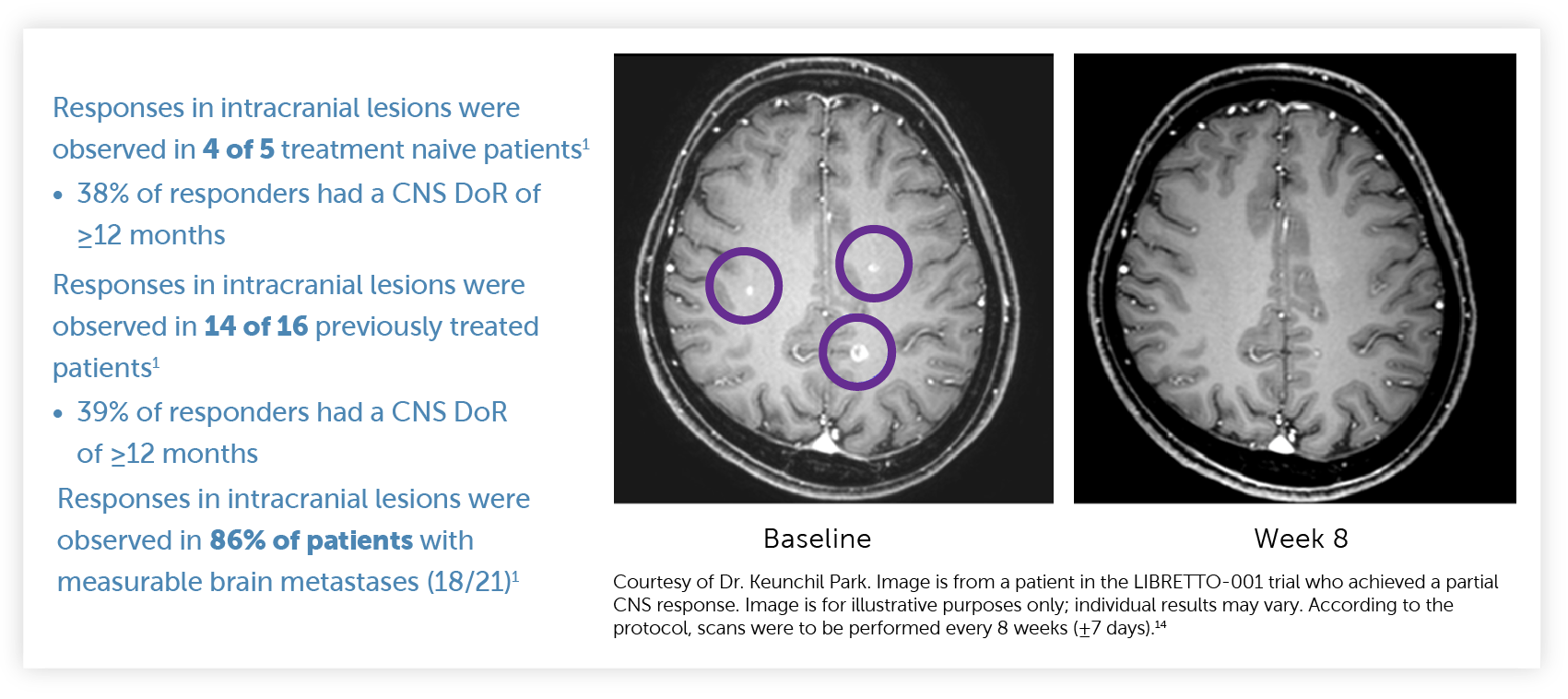
CNS ORR and CNS DoR were prespecified secondary endpoints that were evaluated and confirmed by an IRC.1,15
Select Important Safety Information
Hypertension occurred in 41% of patients, including Grade 3 hypertension in 20% and Grade 4 in one (0.1%) patient. Overall, 6.3% had their dose interrupted and 1.3% had their dose reduced for hypertension. Treatment-emergent hypertension was most commonly managed with anti-hypertension medications. Do not initiate Retevmo in patients with uncontrolled hypertension. Optimize blood pressure prior to initiating Retevmo. Monitor blood pressure after 1 week, at least monthly thereafter, and as clinically indicated. Initiate or adjust anti-hypertensive therapy as appropriate. Withhold, reduce dose, or permanently discontinue Retevmo based on the severity.
Response in patients with advanced or metastatic RET fusion-positive thyroid cancer (non-MTC)1ǁǁ
Systemic therapy-naive¶¶ patients (n=8)1,16
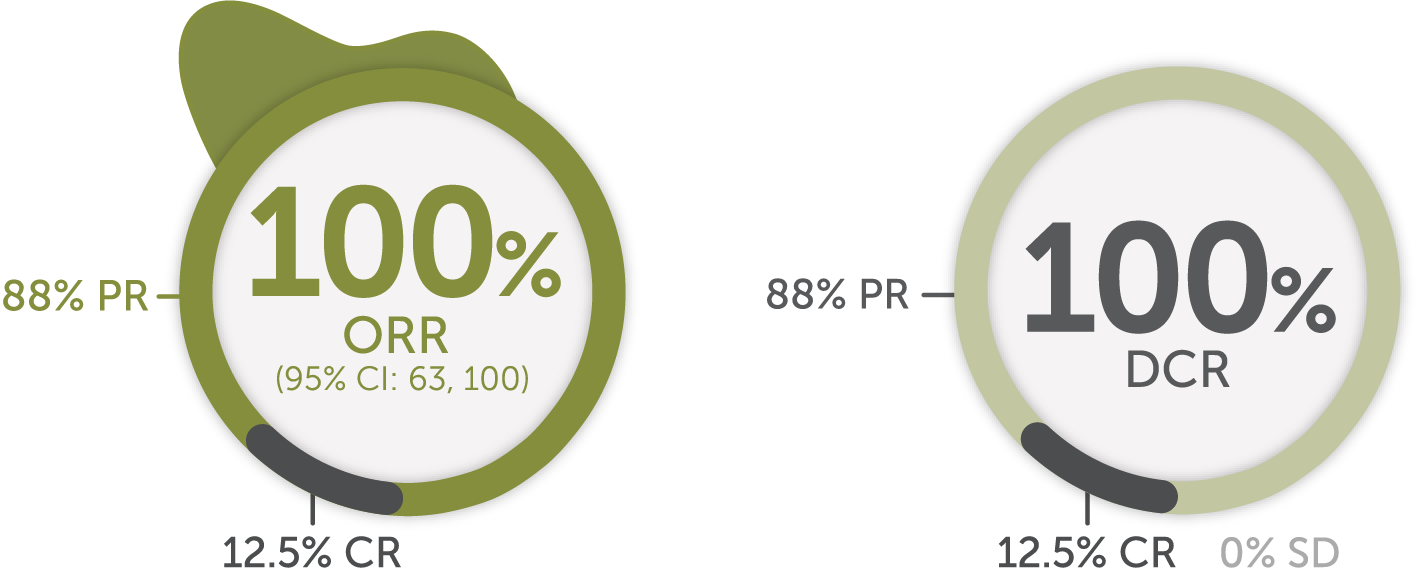
Median DoR not yet reached
(95% CI: NE, NE)
Median follow-up: 8.8 months1,17
The major efficacy outcome measures in LIBRETTO-001 were ORR and DoR. DCR was not a prespecified endpoint and is a post-hoc calculation. DCR is defined as ORR (CR + PR) + SD.
SD is defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, per RECIST v1.1. In the setting of a single-arm trial without the ability to compare with a control arm provided by a randomized trial, the interpretation and clinical relevance of a best overall response of SD are not clear, and it is not possible to determine if SD is a result of natural disease progression or treatment with Retevmo.1,11
Previously treated## patients (n=19)1,16
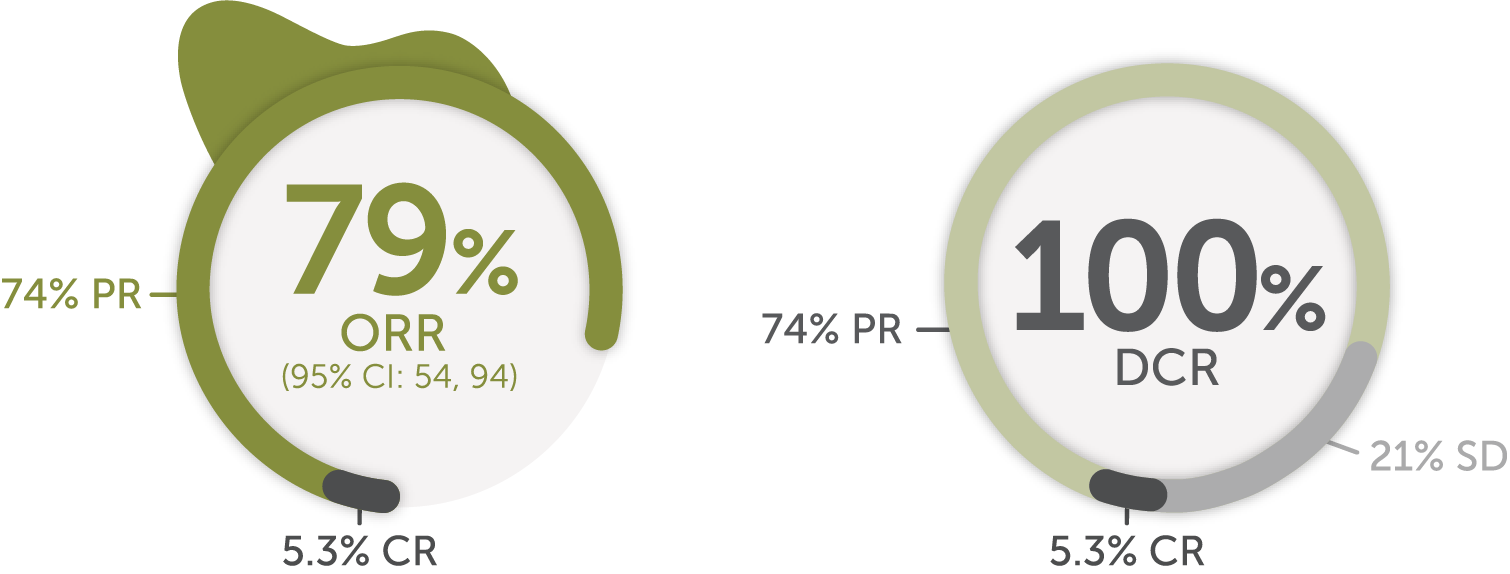
Median DoR
18.4
months
(95% CI: 7.6, NE)
Median follow-up: 17.5 months1,17
All results reviewed by an IRC.1,16,17
ǁǁPrimary tumor histologies included papillary thyroid cancer, poorly differentiated thyroid cancer, anaplastic thyroid cancer, and Hurthle cell thyroid cancer.1
¶¶Patients received no prior systemic therapy other than RAI.1
##Patients received a prior systemic therapy (including sorafenib, lenvatinib, or both) other than RAI.1
Response in patients with advanced or metastatic RET-mutant MTC1
Cabozantinib/vandetanib-naive patients (n=88)1,18
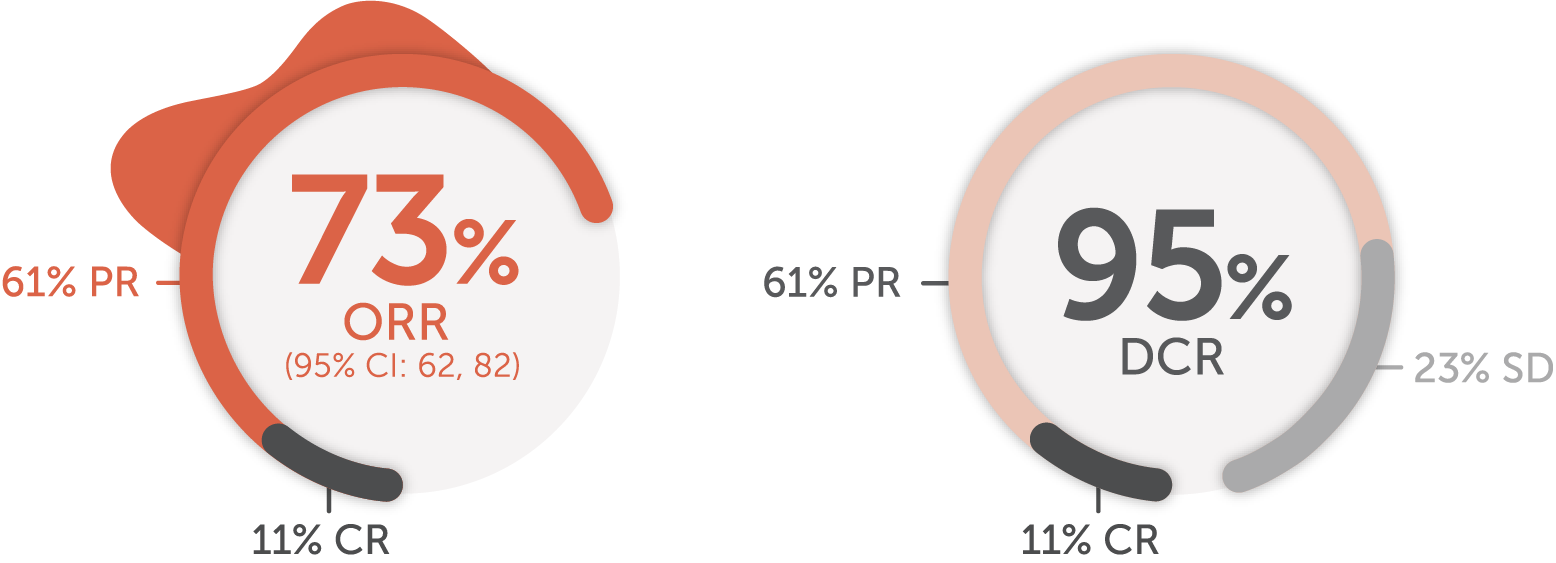
Median DoR
22.0
months
(95% CI: NE, NE)
Median follow-up: 7.8 months1,17
The major efficacy outcome measures in LIBRETTO-001 were ORR and DoR. DCR was not a prespecified endpoint and is a post-hoc calculation. DCR is defined as ORR (CR + PR) + SD.
SD is defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, per RECIST v1.1. In the setting of a single-arm trial without the ability to compare with a control arm provided by a randomized trial, the interpretation and clinical relevance of a best overall response of SD are not clear, and it is not possible to determine if SD is a result of natural disease progression or treatment with Retevmo.1,11
Patients previously treated*** with cabozantinib and/or vandetanib (n=55)1,18
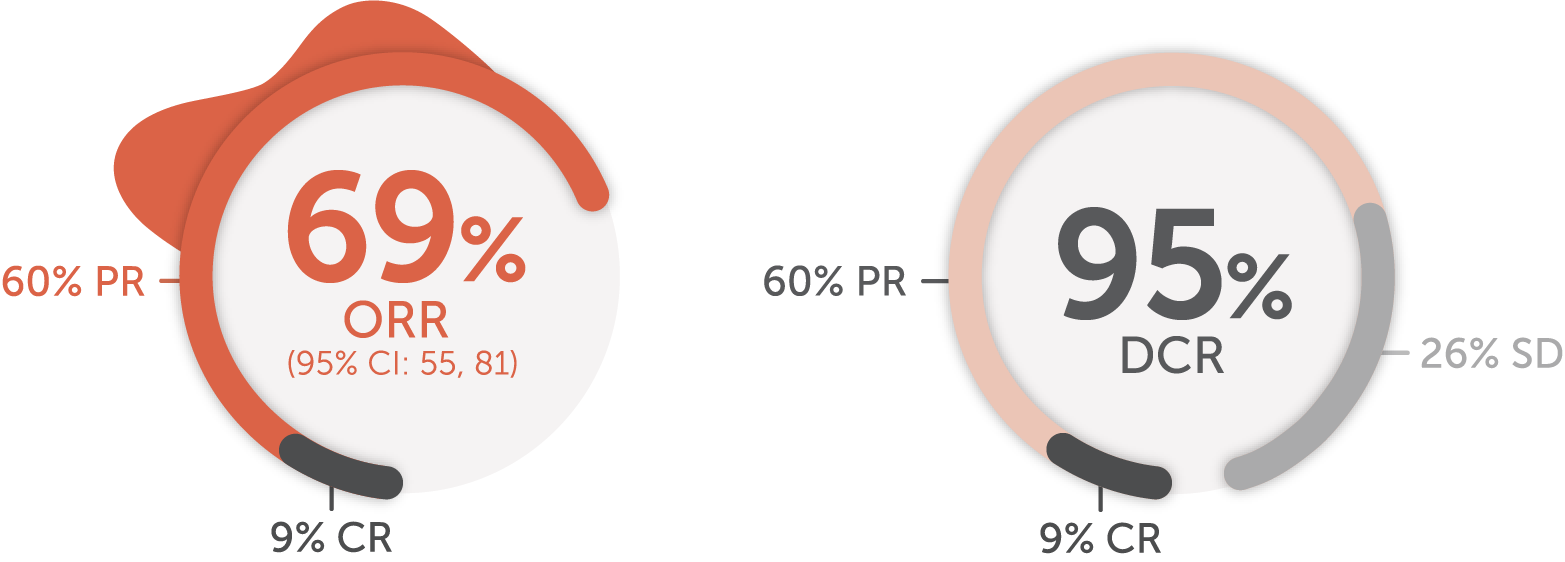
Median DoR not yet reached
(95% CI: 19.1, NE)
Median follow-up: 14.1 months1,17
All results reviewed by an IRC.1,17,18
***The efficacy of Retevmo was evaluated in 55 patients with RET-mutant advanced MTC who were previously treated with cabozantinib or vandetanib enrolled into a cohort of LIBRETTO-001.1
Response in the tumor agnostic cohort in patients with locally advanced or metastatic RET fusion-positive solid tumors1
In the tumor agnostic††† cohort (n=41)1,5:
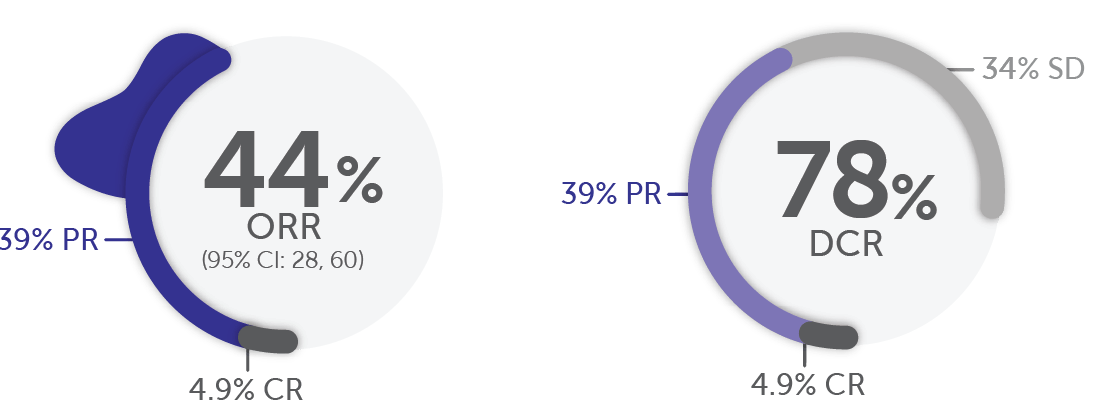
Median DoR
24.5
months
(95% CI: 9.2, NE)
Median follow-up: 14.9 months
DCR was not a prespecified endpoint and is a post-hoc calculation. DCR is defined as ORR (CR + PR) + SD.1
SD is defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, per RECIST v1.1. In the setting of a single-arm trial without the ability to compare with a control arm provided by a randomized trial, the interpretation and clinical relevance of a best overall response of SD are not clear, and it is not possible to determine if SD is a result of natural disease progression or treatment with Retevmo.1,11
All results reviewed by an IRC.1
Due to rounding, numbers presented may not add up to the totals indicated and percentages may not reflect the absolute figures for the same reason.
At the time of analysis (September 24, 2021), 50% of responses (n=9/18) were ongoing.5
†††Efficacy was evaluated in 41 adult patients with advanced or metastatic RET fusion-positive solid tumors. Thirty-seven patients received prior systemic therapy (with a median of 2 prior systemic regimens).1
In RET fusion-positive pancreatic adenocarcinoma, more than half of patients responded to Retevmo (n/N=6/11)1,19
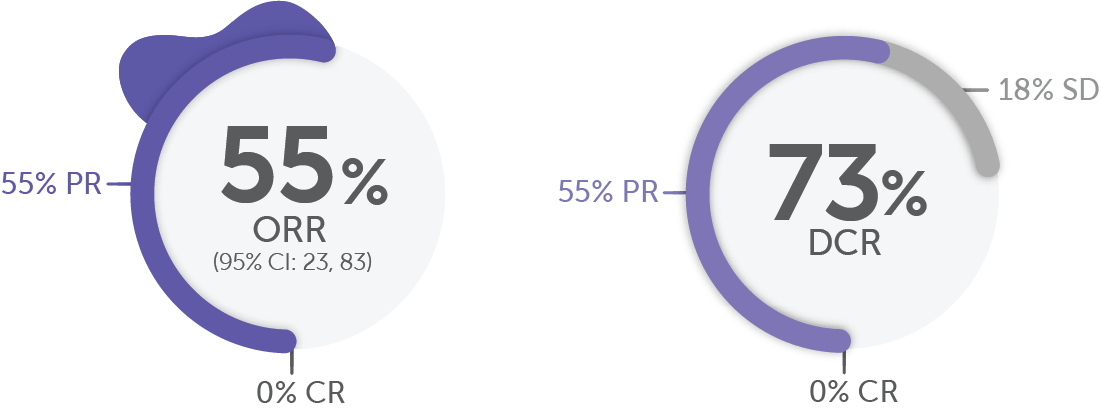
DoR Range
2.5, 38.3+ months
(Based on 6 responders)
+Denotes ongoing response.
Median prior systemic therapies: 2.20
In RET fusion-positive colorectal cancer (n=10)1,19
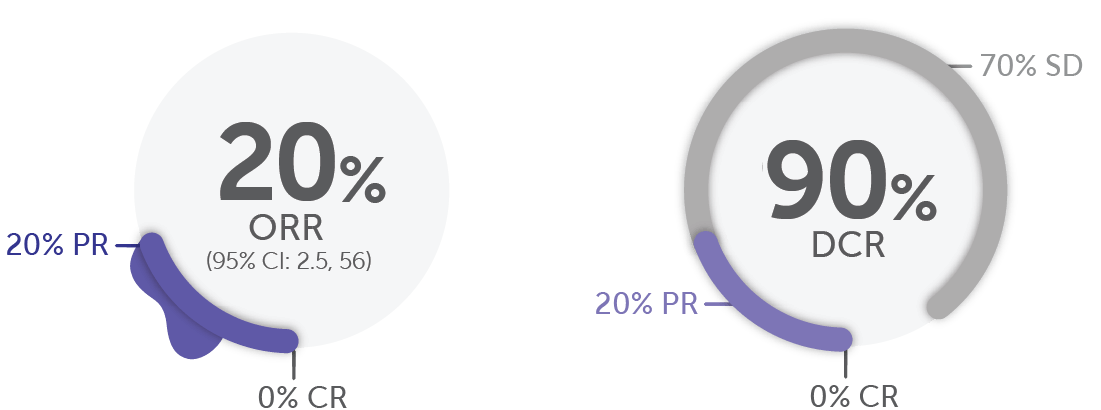
DoR Range
5.6, 13.3 months
(Based on 2 responders)
Median prior systemic therapies: 3.5.20
The major efficacy outcome measures in LIBRETTO-001 were ORR and DoR. DCR was not a prespecified endpoint and is a post-hoc calculation. DCR is defined as ORR (CR + PR) + SD.
SD is defined as neither sufficient shrinkage to quality for PR nor sufficient increase to qualify for PD, per RECIST v1.1. In the setting of a single-arm trial without the ability to compare with a control arm provided by a randomized trial, the interpretation and clinical relevance of a best overall response of SD are not clear, and it is not possible to determine if SD is a result of natural disease progression or treatment with Retevmo.1,11
All results reviewed by an IRC.1,19
Because Retevmo is approved across all lines of therapy, including first line in certain RET-driven cancers, consider waiting for genomic test results, including RET alteration results, before making therapeutic decisions1
Select Important Safety Information
Retevmo can cause concentration-dependent QT interval prolongation. An increase in QTcF interval to >500 ms was measured in 7% of patients and an increase in the QTcF interval of at least 60 ms over baseline was measured in 20% of patients. Retevmo has not been studied in patients with clinically significant active cardiovascular disease or recent myocardial infarction. Monitor patients who are at significant risk of developing QTc prolongation, including patients with known long QT syndromes, clinically significant bradyarrhythmias, and severe or uncontrolled heart failure. Assess QT interval, electrolytes, and thyroid-stimulating hormone (TSH) at baseline and periodically during treatment, adjusting frequency based upon risk factors including diarrhea. Correct hypokalemia, hypomagnesemia, and hypocalcemia prior to initiating Retevmo and during treatment. Monitor the QT interval more frequently when Retevmo is concomitantly administered with strong and moderate CYP3A inhibitors or drugs known to prolong QTc interval. Withhold and dose reduce or permanently discontinue Retevmo based on the severity.

Selpercatinib (Retevmo) is a National Comprehensive Cancer Network® (NCCN®)-preferred (category 2A‡‡‡) treatment option for first-line or subsequent§§§ therapy in patients with RET-positive metastatic non-small cell lung cancer (NSCLC)21ǁǁǁ
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) recommendations for NSCLC20:
Specific targeted therapies are the preferred systemic treatment options for eligible patients with metastatic NSCLC with certain driver alterations.¶¶¶
For patients with metastatic NSCLC who are RET rearrangement positive, NCCN NSCLC Panel recommends selpercatinib (Retevmo) as a first-line or subsequent§§§ therapy option (category 2A‡‡‡) (preferred) for patients with metastatic NSCLC who are positive for RET rearrangements.
NCCN Guidelines® recommendations for RET alteration-positive thyroid cancer22:
Selpercatinib is a recommended systemic therapy option (category 2A‡‡‡) in RET fusion-positive:
- Structurally persistent/recurrent locoregional or distant metastatic (including bone or CNS metastases) papillary carcinoma, follicular carcinoma, or Hurthle carcinoma, that is not amenable to RAI therapy
Selpercatinib is a preferred systemic therapy option (category 2A‡‡‡) in RET fusion-positive:
- Metastatic anaplastic carcinoma###
Selpercatinib is a preferred systemic therapy option (category 2A‡‡‡) in RET mutation-positive medullary carcinoma****:
- Locoregional, unresectable carcinoma that is symptomatic or progressing by RECIST criteria
- Distant metastatic, asymptomatic carcinoma that is unresectable and progressing by RECIST criteria
- Distant metastatic, symptomatic or progressive carcinoma
‡‡‡Category 2A: Based upon lower-level evidence, and there is uniform NCCN consensus that the intervention is appropriate.
§§§If RET inhibitors have not been previously used.
ǁǁǁSee the NCCN Guidelines for NSCLC for detailed recommendations, including other preferred treatment options.
¶¶¶The NCCN Guidelines for NSCLC provide recommendations for individual biomarkers that should be tested and recommend testing techniques but do not endorse any specific commercially available biomarker assays or commercial laboratories.
###Molecular testing should include BRAF, NTRK, ALK, RET, MSI, dMMR, and tumor mutational burden.
****RET somatic genotyping in patients who are germline wild-type or germline unknown.
ALK=anaplastic lymphoma kinase; BRAF=v-raf murine sarcoma viral oncogene homolog B; CI=confidence interval; dMMR=DNA mismatch repair; MSI=microsatellite instability; NE=not estimable; NTRK=neurotrophic receptor tyrosine kinase.
References: 1. Retevmo (selpercatinib). Prescribing Information. Lilly USA, LLC. 2. Phase 1/2 study of LOXO-292 in patients with advanced solid tumors, RET fusion-positive solid tumors, and medullary thyroid cancer (LIBRETTO-001). https://clinicaltrials.gov/ct2/show/NCT03157128. Updated June 9, 2022. Accessed June 14, 2022. 3. Drilon A, Subbiah V, Gautschi O, et al. Durability of efficacy and safety with selpercatinib in patients with RET fusion+ non-small-cell lung cancer: LIBRETTO-001. Poster presented at: European Lung Cancer Congress; March 30–April 2, 2022. Poster 27P. 4. Data on File, Lilly USA, LLC, DOF-SE-US-0063. 5. Subbiah V, Wolf J, Konda B, et al. Tumor agnostic efficacy of selpercatinib in patients with RET fusion-positive solid tumors: a global, multicenter trial updated (LIBRETTO-001). Presented at: 2022 ASCO Annual Meeting; June 3-7, 2022; Chicago. Abstract 3094. 6. Drilon A, Oxnard GR, Tan DSW, et al. Efficacy of selpercatinib in RET fusion–positive non–small-cell lung cancer. N Engl J Med. 2020;383(9):813-824. 7. Wirth LJ, Sherman E, Robinson B, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med. 2020;383(9):825-835. 8. McCoy CE. Understanding the intention-to-treat principle in randomized controlled trials. West J Emerg Med. 2017;18(6):1075-1078. 9. Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res. 2011;2(3):109-112. 10. Data on File, Lilly USA, LLC, DOF-SE-US-0057. 11. Villaruz LC, Socinski MA. The clinical viewpoint: definitions, limitations of RECIST, practical considerations of measurement. Clin Cancer Res. 2013;19(10):2629-2636. 12. Data on File, Lilly USA, LLC, DOF-SE-US-0058. 13. Data on File, Lilly USA, LLC, DOF-SE-US-0028. 14. Data on File, Lilly USA, LLC, DOF-SE-US-0013. 15. Data on File, Lilly USA, LLC, DOF-SE-US-0033. 16. Data on File, Lilly USA, LLC, DOF-SE-US-0031. 17. Data on File, Lilly USA, LLC, DOF-SE-US-0032. 18. Data on File, Lilly USA, LLC, DOF-SE-US-0024. 19. Data on File, Lilly USA, LLC, DOF-SE-US-0069. 20. Data on File, Lilly USA, LLC, DOF-SE-US-0067. 21. Referenced with permission from The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer V5.2022. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed December 1, 2022. To view the most recent and complete version of the guidelines, go online to https://www.nccn.org. 22. Referenced with permission from The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Thyroid Carcinoma V3.2022. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed December 1, 2022. To view the most recent and complete version of the guidelines, go online to https://www.nccn.org.
INDICATIONS
Retevmo is a kinase inhibitor indicated for the treatment of:
- adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with a rearranged during transfection (RET) gene fusion, as detected by an FDA-approved test
- adult and pediatric patients 12 years of age and older with advanced or metastatic medullary thyroid cancer (MTC) with a RET mutation, as detected by an FDA-approved test, who require systemic therapy*
- adult and pediatric patients 12 years of age and older with advanced or metastatic thyroid cancer with a RET gene fusion, as detected by an FDA-approved test, who require systemic therapy and who are radioactive iodine-refractory (if radioactive iodine is appropriate)*
- adult patients with locally advanced or metastatic solid tumors with a RET gene fusion that have progressed on or following prior systemic treatment or who have no satisfactory alternative treatment options*
*These indications are approved under accelerated approval based on overall response rate (ORR) and duration of response (DoR). Continued approval for these indications may be contingent upon verification and description of clinical benefit in confirmatory trials.